性決定の遺伝学 :概念と理論の再考
課題
性決定に関する研究は、以前は主に精巣の発達に焦点が当てられており、卵巣の発達の能動的なプロセスはほとんど無視されていた(Veitia, 2010)。実際、卵巣の発達は、発達段階で男性女性に二分化する生殖腺の「既定」の、または「受動的」なプロセスであると、長い間考えられていた。
方法:概念と理論の再考
女性の発達経路が「既定」であるという考え方によって、精巣の分化に関する研究に焦点が当てられ、Y染色体性決定領域遺伝子(SRY)の発見後はSRYの下流構造にあるSOX9(哺乳類の性分化や軟骨形成に必須の役割を持つ転写因子)などが研究対象になった。その一方で、卵巣の発達経路はあまり研究されなかった。女性の発達経路を「既定」とした科学モデルは、ターナー症候群における卵巣の発達欠如と矛盾していた。
ジェンダード・イノベーション:
- 1. 卵巣形成の能動的プロセスの認識: 現在の研究では、卵巣を形成するのに必要な能動的なメカニズムを明らかにしようとしている(Veitia, 2010; Uhlenhaut et al., 2009)。これらの研究により、精巣の発達と、卵巣経路と精巣経路がどのように相互作用するかについての知識が深まった。
- 2. 卵巣と精巣の維持システムの発見:卵巣経路に関する研究から、転写調節因子FOXL2は、「成体卵巣から精巣への分化転換」を防ぐために、成体卵巣卵胞で発現している必要があることが明らかになった(Uhlenhaut et al., 2009)。続いて、精巣のセルトリ細胞が顆粒膜細胞に再プログラミングされるのを防ぐためには、転写因子DMRT 1が必要であることが明らかなった(Matson et al., 2011)。
- 3. 性腺分化を表現する新しい用語: 研究者らは「既定」の概念を捨て、女性と男性の発達経路は分岐しているが、卵巣の形成も(精巣や他の器官の形成と同様に)能動的なプロセスであることを強調している。それぞれの経路には、適切な量と正確な時期の遺伝子産物の複雑なカスケードが必要である。
The Concept of Female Development as a Hormonal Default
The Concept of Female Development as a Genetic Default
The Challenge: The Idea of Default and its Influence on Research
Gendered Innovation 1: Recognition of Ovarian Determination as an Active Process
Method: Rethinking Concepts and Theories
Gendered Innovation 2: Discovery of Ongoing Ovarian and Testis Maintenance
Gendered Innovation 3: New Language to Describe Gonadal Differentiation
Method: Rethinking Research Priorities and Outcomes
Conclusions
The Concept of Female Development as a Hormonal Default
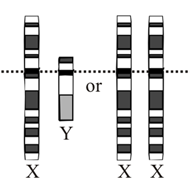
The embryonic gonad is bipotential—that is, it will normally “give rise to one of two morphologically and functionally different organs, a testis or an ovary” (Capel et al., 2006).
In 1947, Alfred Jost demonstrated that, when female (XX) and male (XY) rabbit fetuses are gonadectomized in utero before sexual differentiation, all individuals develop female sex duct structures and female external genitals, regardless of karyotypic sex (Jost, 1947).
Researchers hypothesized that the testes triggered male development through testicular hormones. Studies in cattle showed that, when opposite-sex fetuses have placental anastomoses which allow for the exchange of hormones, XX fetuses are masculinized, but XY fetuses are not feminized (Jost et al., 1972). As a result of these and other studies, the absence of testicular hormones was expected to result in female development.
The Concept of Female Development as a Genetic Default
The 1905 discovery of the Y chromosome by both Nettie Stevens and Edmund Wilson led to the description of an XX/XY sex determination system, in which women were XX and men XY (Stevens, 1905; Wilson, 1905). It was not initially clear whether human sex was determined by the number of X chromosomes or by the presence or absence of the Y chromosome.
Subsequent studies of Klinefelter’s and Turner’s syndromes in the 1950s suggested that the presence of a Y chromosome determines sex in humans (Jacobs et al., 1959; Ford, 1959). If sex were determined by X-chromosome count, (47,XXY) patients with Klinefelter’s syndrome would be expected to be female, since they have the typical X count for women (two), and (45,XO) patients with Turner’s syndrome would be expected to be male, since they have the typical X count for men (one). However, Klinefelter’s syndrome patients have a male phenotype and Turner’s syndrome patients have a female phenotype.
These observations led to a search to find a sex determining gene on the Y-chromosome. In a 1990 Nature paper, Andrew Sinclair and colleagues identified a Y-chromosome gene as the Sex-Determining Region Y (SRY), while acknowledging that it is likely that many different genes are required for both male and female sex determination (Sinclair et al., 1990). Subsequent research confirmed that XX mice develop testes if injected with Sry-bearing DNA fragments during embryonic development (Koopman et al., 1991).
Studies of human patients identified (46,XX) men who had translocations of SRY onto an X-chromosome, further suggesting SRY was sufficient to trigger male development (Berkovitz et al., 1992). In subsequent years, research focused largely on the downstream targets of Sry.
The Challenge: The Idea of Default and its Influence on Research Priorities
In this period, research on sex determination focused on questions concerning the genetics of male testis determination (Richardson, 2013). Female sexual development, by contrast, was thought to proceed as a “default” in the absence of Sry.
The English word, “default,” means “failure to act; neglect” or “a preselected option adopted […] when no alternative is specified” (Oxford English Dictionary, 2011). In the case of sex determination, “default” became the prevailing model for female pathways—i.e., an ovary results in the absence of other action, and ovarian development was understudied.
While the majority of the research community continued to focus on genetics of testis determination as the key to mammalian sexual development, some developmental biologists protested the “default” model. In 1986, for example, Eva Eicher and Linda Washburn challenged the concept of “induction of ovarian tissue as a passive (automatic) event,” arguing that “the induction of ovarian tissues is as much an active, genetically directed developmental process as is the induction of testicular tissue or, for that matter, the induction of any cellular differentiation process.” These biologists noted “almost nothing has been written about genes involved in the induction of ovarian tissue from the undifferentiated gonad” (Eicher et al., 1986; see also Fausto-Sterling, 1989).
Gendered Innovation 1: Recognition of Ovarian Determination as an Active Process
By the mid 1990s, developmental biologists recognized that “although factors involved in male sexual differentiation have been well studied, the pathways regulating female sexual differentiation remain incompletely defined” (see Biason-Lauber et al., 2008; Richardson, 2013).
Simultaneously, data from both animal models and human patients suggested that sex determination involved more than the presence or absence of SRY. Observations include:
- 1. The absence of SRY is not sufficient to build a functioning ovary; two X-chromosomes are required. 45,XO women with Turner syndrome develop ovarian dysfunction, indicating that two X-chromosomes are needed for normal female development (Bondy, 2010). This ovarian dysfunction is caused by loss of germ cells during development. Viable germ cells are required to construct a functioning ovary (Persani et al., 2009). Testicular development differs in that a “functioning” (hormone-secreting) testis can develop in the absence of germ cells, as in the case of XX males (Kim et al., 2010).
- 2. Dosage-sensitive genes can override male development even in the presence of SRY. In 1994, researchers identified (46,XY) women with intact SRY, and determined that duplications of a specific X-chromosome locus “are sufficient to disrupt normal testis development in the presence of SRY” (Bardoni et al., 1994). Later studies identified the gene involved as DAX1, and studies of (46,XY) women showed that “DAX1 duplications in XY individuals cause male-to-female sex reversal” (Ludbrook et al., 2004). As such, DAX1 “can act as an anti-testis gene” (Sekido et al., 2009).
Reconceptualizing the ovarian pathway as “active” yielded an important gendered innovation: Researchers began identifying specific mechanisms required to produce and maintain the ovary—during development, postnatally, and into adulthood. Several genetic candidates emerged, including WNT4 and FOXL2. Researchers came to understand that female sex differentiation requires ongoing maintenance throughout adulthood—see Method. Some genes, such as WNT4, are specifically required for female sex development but not for male sex development (Swain et al., 1998).
Current work suggests that both the male and female pathways rely on dominantly acting genes, with SRY actively promoting the male pathway by upregulating SOX9 expression, while B-catenin, Rspo1, and Foxl2 actively promote the female pathway by repressing SOX9. It is a matter of timing (and expression level) that determines which pathway prevails (Sekido et al., 2008; Veitia, 2010)—see Figure.

Method: Rethinking Concepts and Theories
Theories and concepts are one factor framing research priorities. In the case of the genetics of sex determination, biologists failed to question the “default” model for ovarian development inherited from the 1950s and 1960s. The notion of a “passive” female process fit with current scientific theories and gender assumptions in the broader society (Schiebinger, 1989; Richardson, 2013). Rethinking theory led to new questions about ovarian development and the discovery of a cohort of genes required for ovarian function. Numerous “gene screening experiments have shown that many genes are expressed specifically in [the] ovary” (Liu, 2010).
Gendered Innovation 2: Discovery of Ongoing Ovarian and Testis Maintenance
In addition to ovarian development, researchers sought to understand specific pathologies of the ovary. Biologists studying the genetics of blepharophimosis / ptosis / epicanthus inversus syndrome (BPES), associated with ovarian failure, identified the gene FOXL2 as necessary for ovarian maintenance (Crisponi, 2001). Later research showed that, in adults, FOXL2 is required to continuously suppress SOX9 and thereby prevent ovarian follicle cells from trandifferentiating into “testis-like” cells (Uhlenhaut et al., 2009)—see diagram below, reproduced from Uhlenhaut et al., 2009.

As occurred with FOXL2, later experiments showed “that male sex determination is not a permanent choice and that Dmrt1 is crucial for maintenance of testicular function” (Herpin et al., 2011). Similar to how loss of FOXL2 can reprogram ovarian granulosa cells into testicular Sertoli cells, loss of DMRT1 can reprogram Sertoli cells into granulosa cells. DMRT1 suppresses certain genes involved in ovarian development—see diagram below, reproduced from Matson et al., 2011.

Gendered Innovation 3: New Language to Describe Gonadal Differentiation
Ovarian determination is no longer seen as a “default” process such that the absence of SRY automatically leads to the development of an ovary. Rather researchers describe both pathways as active, requiring complex cascades of gene products in proper dosages and at precise times—see Method.
Method: Rethinking Research Priorities and Outcomes
In this case, rethinking concepts and theories led to rethinking research priorities. While research priorities in the genetics of sex determination from the 1940s through the 1990s privileged study of genes involved in the induction of testis tissue from the undifferentiated gonad, little research focused on the ovarian pathway. Although many questions remain, researchers have now begun to identify the active mechanisms required to produce and maintain the ovary. This has led, in turn, to new research into the maintenance of testicular function.
Conclusions
Ovarian development is clearly not a default or passive pathway. Biologists, geneticists, and other researchers have recognized that understanding ovarian development is critical to understanding the genetics of sex determination. New research on the active ovarian pathway has led to changes in language used to describe sex determination—current language emphasizes the gene-driven nature of both ovarian and testis formation.
Works Cited
Bardoni, B., Zanaria, E., Guioli, S., Floridia, G., Worley, K., Tonini, G., Ferrante, E., Chiumello, G., McCabe, E., Fraccaro, M., Zuffardi, O., & Camerino, G. (1994). A Dosage Sensitive Locus at Chromosome X021 is Involved in Male to Female Sex Reversal. Nature Genetics, 7 (4), 497-501.
Berkovitz, G., Fechner, P., Marcantonio, S., Bland, G., Stetten, G., Goodfellow, P., Smith, K., & Migeon, C. (1992). The Role of the Sex-Determining Region of the Y Chromosome (SRY) in the Etiology of 46,XX True Hermaphroditism. Human Genetics, 88 (4), 411-416.
Biason-Lauber, A., & Konrad, D. (2008). WNT4 and Sex Development. Sexual Development, 2 (4-5), 210-218.
Bondy, C. (2010). Turner Syndrome. In Carrell, D., & Peterson, C. (eds.), Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice, pp. 307-324. New York: Springer Science and Business Media.
Brennan, J., & Capel, B. (2004). One Tissue, Two Fates: Molecular Genetic Events that Underlie Testis versus Ovary Development. Nature Reviews Genetics, 5 (7), 509-521.
Capel, B., & Kim, Y. (2006). Balancing the Bipotential Gonad between Alternative Organ Fates: A New Perspective on an Old Problem. Developmental Dynamics, 235 (9), 2292-2300.
Cotinot, C., Pailhoux, E., Jaubert, F., & Fellous, M. (2002). Molecular Genetics of Sex Determination. Seminars in Reproductive Medicine, 20 (3), 157-168.
Crisponi, L., Deiana, M., Loi, A., Chiappe, F., Uda, M., Amati, P., Bisceglia, L., Zelante, L., Nagaraja, R., Porcu, S., Ristaldi, M., Marzella, R., Rocchi, M., Nicolino, M., Leinhardt-Roussie, A., Nivelon, A., Verloes, A., Schlessinger, D., Gasparini, P., Bonneau, D., Cao, A., & Pilia, G. (2001). The Putative Forkhead Transcription Factor FOXL2 is Mutated in Blepharophimosis/Ptosis/Epicanthus Inversus Syndrome. Nature Genetics, 27 (2), 159-166.
DiNapoli, L., & Capel, B. (2008). SRY and the Standoff in Sex Determination. Molecular Endocrinology, 22 (1), 1-9.
Eicher, E., & Washburn, L. (1986). Genetic Control of Primary Sex Determination in Mice. Annual Review of Genetics, 20, 327-60.
Fausto-Sterling, A. (1989). Life in the XY Corral. Women’s Studies International Forum, 12 (3), 319-331.
Ford, C., Miller, O., Polani, E., de Almeida, J., & Briggs, J. (1959). A Sex Chromosome Anomaly in a Case of Gonadal Dysgenesis (Turner’s Syndrome). Lancet, 1 (7075), 711-713.
Herpin, A., & Schartl, M. (2011). Sex Determination: Switch and Suppress. Current Biology, 21 (17), R656-R659.
Jacobs, P., & Strong, J. (1959). A Case of Human Intersexuality Having a Possible XXY Sex-Determining Mechanism. Nature, 4657 (183), 302-303.
Jost, A. (1972). A New Look at the Mechanisms Controlling Sex Differentiation in Mammals. Johns Hopkins Medical Journal, 130 (1), 38-53.
Jost, A. (1970). Hormonal Factors in the Sex Differentiation of the Mammalian Foetus. Philosophical Transactions of the Royal Society of London, 259, 119-130.
Jost, A. (1947). Recherches sur la Différenciation Sexuelle de l'Embryon de Lapin. Archives d'Anatomie Microscopique et de Morphologie Expérimentale, 36, 271–315.
Kim, J., Bak, C., Chin, M., Cha, D., Yoon, T., & Shim, S. (2010). SRY-Negative 46,XX Infertile Male with Leydig Cell Hyperplasia: Clinical, Cytogenetic, and Molecular Analysis and Review of the Literature. Fertility and Sterility, 94 (2), 753e5-753e9.
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P., & Lovell-Badge, R. (1991). Male Development of Chromosomally Female Mice Transgenic for SRY. Nature, (351), 117-121.
Liu, C. (2010). The Role of Beta-Catenin in the Development of Fetal Ovary and Female Germ Cells. Urbana-Champaign: Illinois Digital Environment for Access to Learning and Scholarship (IDEALS).
Loffler, K., Zarkower, D., & Koopman, P. (2003). Etiology of Ovarian Failure in Blepharophimosis Ptosis Epicanthus Inversus Syndrome: Foxl2 Is a Conserved, Early-Acting Gene in Vertebrate Ovarian Development. Endocrinology, 144 (7), 3237-3243.
Ludbrook, L., & Harley, V. (2004). Sex Determination: A ‘Window’ of DAX1 Activity. Trends in Endocrinology and Metabolism, 15 (3), 116-121.
Matson, C., Murphy, M., Sarver, A., Griswold, M., Bardwell, V., & Zarkower, D. (2011). DMTR1 Prevents Female Reprogramming in the Postnatal Mammalian Testis. Nature, 476 (7358), 101-105.
Oxford English Dictionary. (2011). Default (Noun).
Persani, L., Rossetti, R., Cacciatore, C. & Bonomi, M. (2009). Primary Ovarian Insufficiency: X Chromosome Defects and Autoimmunity. Journal of Autoimmunity, 33 (1), 35-41.
Richardson, S. (2013). Sex Itself: The Search for Male and Female in the Human Genome. Chicago: The University of Chicago Press.
Schiebinger, L. (1989). The Mind Has No Sex? Women in the Origins of Modern Science. Cambridge: Harvard University Press.
Schmidt, D., Ovitt, C., Anlag, K., Fehsenfeld, S., Gredsted, L., Treier, A., & Treier, M. (2004). The Murine Winged-Helix Transcription Factor Foxl2 is Required for Granulosa Cell Differentiation and Ovary Maintenance. Development, 131 (4), 933-942.
Sekido, R., & Lovell-Badge, R. (2009). Sex Determination and SRY: Down to a Wink and a Nudge? Trends in Genetics, 25 (1), 19-29.
Sekido, R., & Lovell-Badge, R. (2008). Sex Determination Involves Synergistic Action of SRY and SF1 on a Specific Sox9 Enhancer. Nature, 453, 930-934.
Shaikh, M., Boyes, L., Kingston, H., Collins, R., Besley, G., Padmakumar, B., Ismayl, O., Hughes, I., Hall, C., Hellerud, C., Achermann, J., & Clayton, P. (2007). Skewed X Inactivation is Associated with Phenotype in a Female with Adrenal Hypoplasia Congenita. Journal of Medical Genetics, 45 (9), 1-5.
Sinclair, A., Berta, P., Palmer, M., Hawkins, J., Griffiths, B., Smith, M., Foster, J., Frischauf, A., Lovell-Badge, R., & Goodfellow, P. (1990). A Gene from the Human Sex-Determining Region Encodes a Protein with Homology to a Conserved DNA-Binding Motif. Nature, 346, 240-245.
Stevens, N. (1905). A Study of the Germ Cells of Aphis Rosae and Aphis Oenotherae. Journal of Experimental Zoology, 2 (3), 313-333.
Swain, A., Narvaez, V., Burgoyne, P., Camerino, G., & Lovell-Badge, R. (1998). Dax1 Antagonizes Sry Action in Mammalian Sex Determination. Nature, 391 (6669), 761-767.
Tannour-Louet, M., Han, S., Corbett, S., Louet, J., Yatsenko, S., Meyers, L., Shaw, C., Kang, S., Cheung, S., & Lamb, D. (2010). Identification of De Novo Copy Number Variants Associated with Human Disorders of Sexual Development. Public Library of Science (PLoS) Biology, 5 (10), e15392, 1-13.
Turnbull, C., Rapley, E., Seal, S., Pernet, D., Renwick, A., Hughes, D., Ricketts, M., Linger, R., Nsengimana, J., Deloukas, P., Huddart, R., Bishop, D., Easton, S., Stratton, M., Rahman, N., & The United Kingdom Testicular Cancer Collaboration (2010). Variants near DMRT1, TERT, and ATF7IP are Associated with Testicular Germ Cell Cancer. Nature Genetics, 42 (7), 604-607.
Uhlenhaut, N., Jakob, S., Anlag, K., Eisenberger, T., Sekido, R., Kress, J., Treier, A., Klugmann, C., Klasen, C., Holter, N., Riethmacher, D., Schütz, G., Cooney, A., Lovell-Badge, R., & Treier, M. (2009). Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell, 139 (6), 1130-1142.
Veitia, R. (2010). FOXL2 Versus SOX9: A Lifelong “Battle of the Sexes.” BioEssays, 32 (5), 375-380.
Vilain, E. (2009). X-Linked Adrenal Hypoplasia Congenita. In Pagon, R., Dolan, C., & Stephens, K. (eds.), Gene Reviews. Seattle: University of Washington Press.
Welt, C. (2007). Primary Ovarian Insufficiency: A More Accurate Term for Premature Ovarian Failure. Clinical Endocrinology, 68 (4), 499-509.
Wilson, E. (1905). The Chromosomes in Relation to the Determination of Sex in Insects. Science, 22 (564), 500-502.
Research on sex determination (the differentiation of the embryonic bipotential gonad into a testis or an ovary) traditionally focused on testis development. Andrew Sinclair’s 1990 Nature paper famously identified a Y-chromosome gene as the Sex-Determining Region Y (SRY). Female sexual development, by contrast, was thought to proceed as a "default" in the absence of Sry. In the case of sex determination, "default" became the prevailing concept for female pathways—i.e., an ovary results in the absence of other action. The active processes controlling ovarian development remained a blind spot. The notion of a "passive" female fit with current scientific theories and gender assumptions in the broader society.
Gendered Innovations:
Around 2010, questioning the notion of "default" led to the discovery of a cohort of genes required for ovarian function. Gender analysis led to three innovations in this field:
- 1. Recognition of ovarian determination as an active process. These investigations have also enhanced knowledge about testis development, and how the ovarian and testicular pathways interact (see chart).
- 2. Discovery of ongoing ovarian and testis maintenance. Research into the ovarian pathway revealed that the transcriptional regulator FOXL2 must be expressed in adult ovarian follicles to prevent "transdifferentiation of an adult ovary to a testis." Subsequently, researchers found that the transcription factor DMRT1 is needed to prevent reprogramming of testicular Sertoli cells into ovarian granulosa cells.
- 3. New language to describe gonadal differentiation. Researchers have dismissed the concept of "default" and emphasize that, while female and male developmental pathways are divergent, the construction of an ovary (like the construction of a testis or any other organ) is an active process. Each pathway requires complex cascades of gene products in proper dosages and at precise times.