処方薬:セックスとジェンダーの分析
課題
歴史的に、医薬品開発は「全ての患者を1つの商品で対応する」というモデルで行われてきた。薬物試験は、齧歯類の前臨床研究から臨床試験に至るまで、主に男性を対象として実施されてきた(Mazure & Jones, 2015)。その結果、望ましくない、または命にかかわる副作用の報告は、男性よりも女性に多くなっている(US GAO, 2001)。
方法:セックス分析
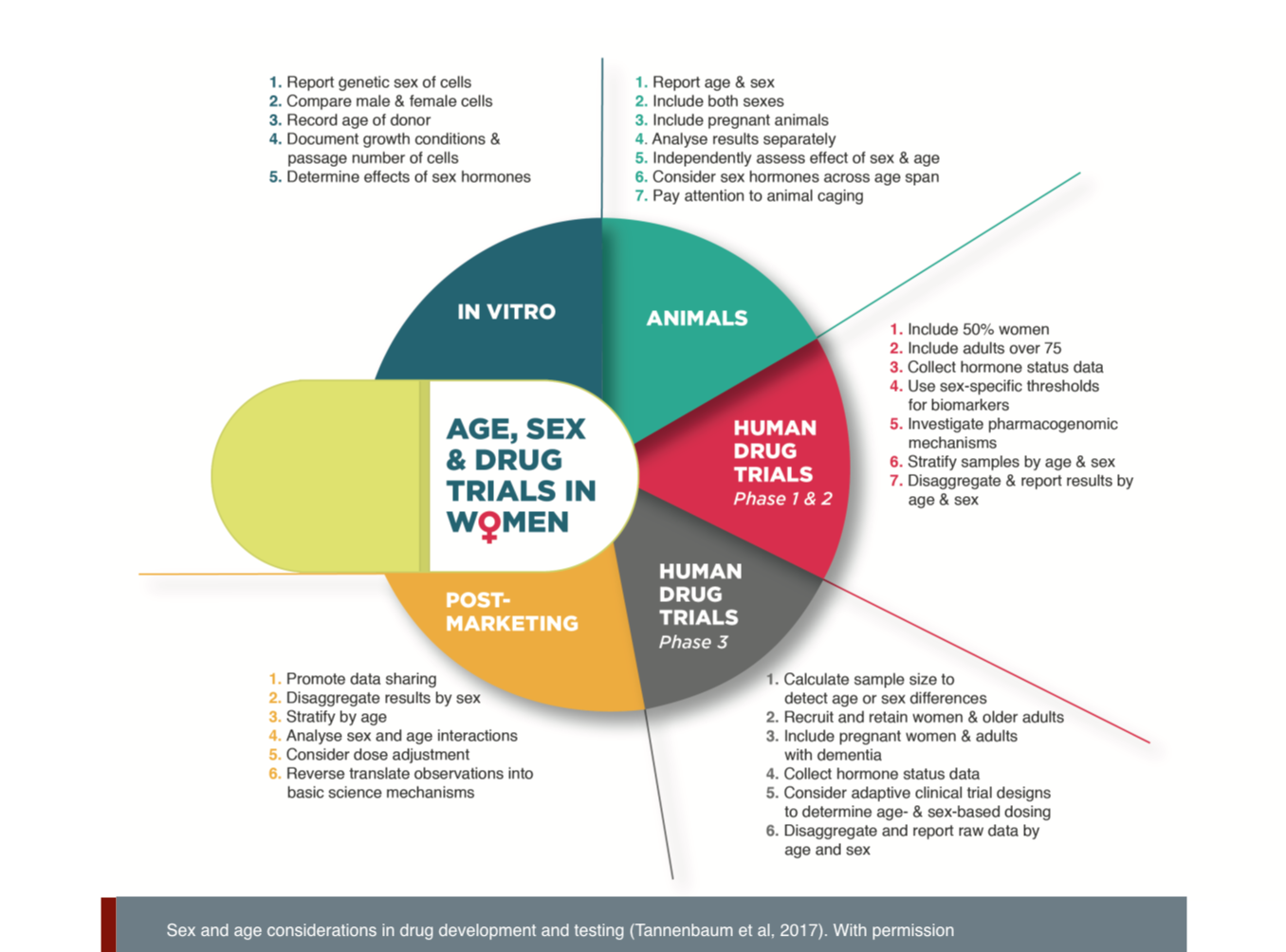
初期の細胞・動物実験からヒト臨床試験に至るまで、すべての医薬品試験にメスとオス、女性と男性を含め、別々に分析する必要がある(Tannenbaum et al., 2017)。また、年齢と遺伝的素質も、薬効、安全性、毒性に影響を与える可能性があるため、考慮する必要がある(Dandara et al.,2014)。妊婦が病気になったり、病気の女性が妊娠したり、妊娠にも注意を払う必要がある。胎児に対する医薬品の安全性への影響に関する情報が優先される。
方法:ジェンダー分析
ジェンダー・ステレオタイプは、いくつかの点で臨床ケアに影響を与える可能性がある。第一に、ジェンダーや年齢のバイアスが診断のための質問に影響を与えることがある(Sheehan & Tucker-Drob, 2017; Verdam et al., 2017)。医師や研究者は、診断の際の言葉遣いとジェンダー規範に注意を払う必要がある。第二に、医療提供者と患者のジェンダーは、処方パターンと治療結果に影響を及ぼす可能性がある(Rochon et al., 2918; Greenwood et al., 2018)。また、年齢や人種も関係することがある。
ジェンダード・イノベーション:
1. 医薬品開発のすべての段階に女性と男性を含めることで、有効性、毒性、安全性における性差を理解することができる。これにより、必要に応じて性別に応じた服用量や治療法を開発することが可能になる。
2. 副作用報告の性別による分類は、臨床での処方において、薬の作用が女性と男性でどのように異なるかについての理解を深めるために不可欠なステップとなる。
3. 医薬品ラベルへの性差情報の記載は、処方者や患者が適切な薬剤を適切な患者に適切な用量で適切な時期に投与するのに役立つ。
4. ジェンダー変革的な治療アプローチの推進により、診断上の問題におけるジェンダーバイアスとステレオタイプを排除し、女性、男性、多様な性の人々の症状をよりよく検出することができる。
The Challenge
Historically, drug development has followed a “one-size-fits-all” model. Drug testing has been conducted predominantly on males from preclinical research in rodents to clinical trials that include few women (Mazure & Jones, 2015). As a result, women report more unwanted, and sometime deadly, side effects than men. In the late 1990s, ten drugs were withdrawn from US markets. Of these, eight showed greater health risks for women (US GAO, 2001).
Gendered Innovation 1: Including Females and Males in all Stages of Drug Development
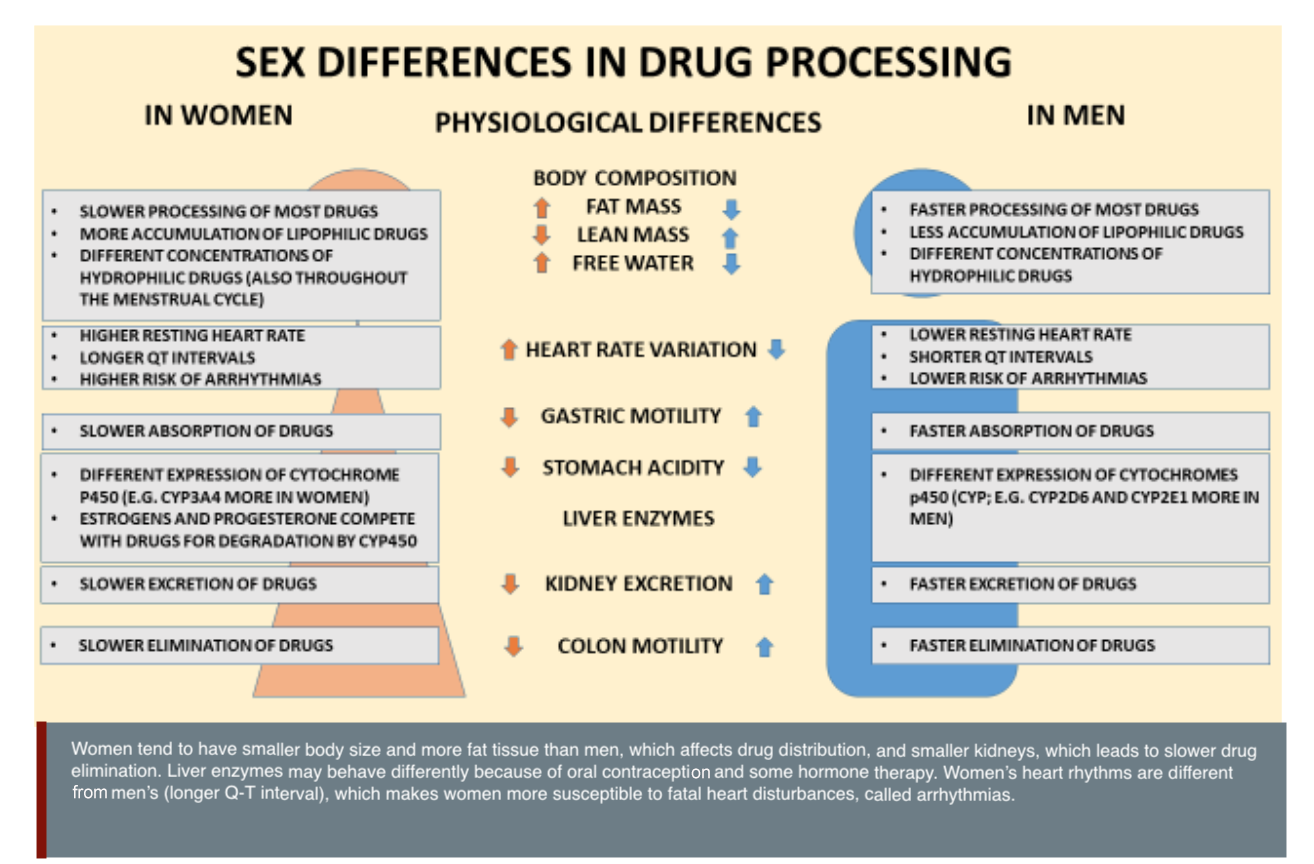
Female and male bodies metabolize drugs differently (Mauvais-Jarvis et al., 2021). For example, female bodies tend to take longer to digest food and to expel waste through their kidneys. Female and male livers process proteins and other molecules at different speeds (Franconi & Campesi, 2014). Because female-typical and male-typical bodies process drugs at different speeds, people with female-typical bodies may be at higher risk of side effects and overdose than people with male-typical bodies (Freire et al., 2011).The two main types of medications are small molecules (that bind to receptors in the body) and biologics (proteins, antibodies, and other substances already found in humans). Small molecules may have sex-specific pharmacokinetics (how the body metabolizes the drug) and pharmacodynamics (how the drug affects the body—see figure). Female-typical bodies and male-typical bodies may also respond differently to biologics since the production and function of immune cells and antibodies can be affected by an individual’s sex chromosomes and levels of estrogens and androgens.
Recognizing these problems, the US created the Food and Drug Administration Office of Women’s Health (FDA-OWH) in 1994 to advocate for women’s inclusion in clinical trials (Obias-Manno et al., 2007). The 1993 US NIH Revitalization Act also mandates the inclusion of women and minorities in clinical trials (Mastroianni et al., 1994). Despite these important steps, female cells, animals and human participants are still not properly included in all stages of the drug development process. The British Journal of Pharmacology recommends that all future studies either include both sexes in the experimental design or explain why researchers believe sex and gender are not relevant (Docherty et al., 2019). Other journals and funding agencies have developed similar policies to promote sex analysis in drug development.
Method: Analyzing Sex in Tissues and Cells
Female and male cells are affected by their sex chromosomes and by the hormonal influences in their environment. Furthermore, certain types of cells, e.g. liver cells, produce different amounts of metabolic enzymes. If sex differences are not taken into account, experimental results will probably be irreproducible. Analyzing the cell response to medications in a sex-specific manner can offer early indications of potential differences that should influence the drug development process (Docherty et al., 2019).
Gendered Innovation 2: Disaggregating Side Effects Reporting by Sex
Women experience more unwanted drug side effects than men (Spoletini et al., 2012). The magnitude of the problem, however, is difficult to quantify because, historically, countries have not included sex information in their statutory reports of side effects.
New reporting guidelines, such as PRISMA-Equity Extension (Welch et al., 2012) and CONSORT-Equity 2017 (Welch et al., 2017), advocate the reporting of data disaggregated by sex. These guidelines (in addition to more stringent journal reporting requirements noted above) have significantly increased the availability of sex-disaggregated data needed for large comparative studies and for meta-analyses aimed at predicting unwanted side effects better.
Gendered Innovation 3: Reporting Sex Differences on Drug Labels
Some medication already has sex-specific dosing. Take, for example, the drug desmopressin, which activates vasopressin receptors in the kidney to regulate water homeostasis (Juul et al., 2011). Women have been found to be more sensitive to the anti-diuretic effects of vasopressin than men. This may be because the gene for the arginine vasopressin receptor is found on the X chromosome in a region likely to escape X-inactivation. Males have only one X chromosome and therefore only one copy of the vasopressin receptor gene per cell; females are more likely to have two copies. As a result, older women taking desmopressin are most likely to experience a reduction of sodium concentration, leading to side effects such as weakness, dizziness and fainting. To avoid unnecessary harm, both the European Union and Canada have recommended lower dosages of desmopressin for older women. The drug is consequently marketed with different recommended doses on the labelling package for women and men.
Research on biologics shows these new treatments can also have sex-specific effects. For instance, the EU-funded ABIRISK study found sex and age differences in the production of anti-drug antibodies to biologics developed for the treatment of multiple sclerosis (Bachelet et al., 2016). Similarly, patients with melanoma and lung cancer responded differently to checkpoint inhibitor drugs based on their sex. A higher proportion of male than female patients achieved successful remission, depending on the type of biologic used (Conforti et al., 2018). Designed to outsmart cancer cells’ defense tactics, checkpoint inhibitors stimulate natural killer immune cells to attack tumor cells. Natural killer cells are sensitive to estrogen and testosterone, which may explain the observed sex differences (Giefing-Kröll et al., 2015). The EU-funded G-DEFINER project is currently studying the existence of sex-driven differences in immune responses as potential factors contributing to disease outcome and response to immunotherapy.
Method: Analyzing Sex
Females and males must be included in all drug trials—from early cell and animal testing through human clinical trials—and analyzed separately (Tannenbaum et al., 2017). Age and genetic ancestry should also be considered, as these characteristics may affect drug efficacy, safety and toxicity (Dandara et al. 2014). Pregnancy also requires attention, as pregnant women get sick, and sick women get pregnant. Information about drug safety effects on the fetus is a priority.
Gendered Innovation 4: Promoting Gender-Transformative Treatment Approaches
“Gender-transformative” approaches address the causes of gender-based inequities and seek to transform harmful gender norms, roles and relations (WHO, 2011). Consider that anti-anxiety and anti-depressant drugs are prescribed more frequently to women, even though men are more likely to commit suicide. Is it because women behave differently, seeking healthcare services more often? Or do gender norms dictate how women and men are socialized to express feelings and emotions? Do the screening tools prescribers use to detect symptoms exhibit gender bias, preferentially asking about crying and helplessness? The result is a prescription regime that differs not only by sex but as a result of gender norms, behaviors and relations (Rochon et al., 2018). Raising awareness about gender bias in the expression of symptoms, screening questions and patterns of prescribing will help prevent harm.
Gender bias in prescribing can affect men and women. Older women are more likely to receive inappropriate prescriptions both because of age and sex differences in pharmacokinetics and because of gendered patterns in prescribing (Morgan et al., 2016; Sramek et al., 2016; Tannenbaum et al., 2009). This is especially true among women diagnosed with dementia (Moga et al., 2017). Sometimes, however, the situation is reversed: when taking antipsychotic medication for the first time, subsequent hospitalization and death rates among men with dementia exceed those in women. The use of antipsychotic and other medications can rapidly lead to the prescription of a second medication to treat the side effects of the first drug—called a prescribing cascade. To help prevent prescribing cascades, sex and gender differences need to be understood. This is the aim of the GENDERNet Plus-funded project iKascade, which is evaluating how men and women may react to an adverse event differently and to what extent they fall victim to prescribing cascades.
Once gender bias is recognized, solutions should be implemented to narrow gaps in adverse outcomes (Tannenbaum et al., 2016). In the case of heart disease, it is well known that women experience symptoms of heart attack differently from men, leading to underdiagnoses in women (see Heart Disease in Diverse Populations). To redress this inequity, emergency physicians developed a 4-step gendered protocol to bypass many of the gender biases in symptoms, testing, diagnosis, and access to treatment (Hudded et al., 2018). This study found that this protocol, coupled with improved access to treatment, cut mortality rates for women in half.
Method: Analyzing Gender in Health and Biomedicine
Gender stereotypes can affect clinical care in several ways. First, gender and age bias can affect the diagnostic questions asked to detect health conditions (Sheehan & Tucker-Drob, 2017; Verdam et al., 2017). Physicians and researchers should pay attention to language and gender norms in diagnostic procedures. Second, gender relations between the healthcare provider and the patient can affect prescribing patterns and treatment outcomes (Rochon et al., 2018; Greenwood et al., 2018). Age and race can also play a role.
Conclusions
Addressing sex and gender in drug prescribing can start with relatively simple yet powerful steps, from including female and male cells, tissues and organisms throughout the testing process to reporting all data disaggregated by sex. These approaches will provide the knowledge needed to develop sex-specific dose adaptations of existing drugs, such as desmopressin or zolpidem, and may lead to sex-specific treatments in the future. Physicians, pharmacists and patients should consider gender differences in how symptoms are experienced and expressed. What is reported and how it is reported impacts which diagnostic steps occur and which medications are prescribed.
Next Steps
To accelerate these developments:
1. Regulatory agencies should require (not simply recommend) sex-disaggregated reporting of all drug trial results by the pharmaceutical industry. Regulatory agencies should also ensure that sex-specific information is available to prescribers and patients on websites and drug labels.
2. Post-marketing surveillance studies should aim to detect sex-specific side effects and differences in response. Many pharmaceuticals currently on the market were tested and approved in years when women were excluded from clinical trials. Post-marketing surveillance is the only way to obtain data on sex differences in efficacy and toxicity in these drugs.
3. Researchers and physicians should further investigate gendered norms, identity and relations in medical communication and prescription outcomes.
Works Cited
Bachelet, D., Hässler, S., Mbogning, C., Link, J., Ryner, M., Ramanujam, R...ABIRISK Consortium. (2016). Occurrence of anti-drug antibodies against interferon-beta and natalizumab in multiple sclerosis: a collaborative cohort analysis. PLoS One, 11(11: e0162752). doi: 10.1371/journal.pone.0162752.
Conforti, F., Pala, L, Bagnardi, V., De Pas, T., Martinetti, M., Viale, G….Goldhirsch, A.P. L. (2018). Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncology, 19(6), 737-746. https://doi.org/10.1016/S1470-2045(18)30261-4.
Dandara, C., Swart, M., Mpeta, B., Wonkam, A., Masimirembwa, C. (2014). Cytochrome P450 pharmacogenetics in African populations: implications for public health. Expert Opinion on Drug Metabolism & Toxicology, 10(6), 769-785. https://doi.org/10.1517/17425255.2014.894020.
Docherty, J. R., Stanford, S. C., Panattieri, R. A., Alexander, S. P., Cirino, G., George, C. H., ... & Ahluwalia, A. (2019). Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. British Journal of Pharmacology, 176, 4081-4086 doi:10.1111/bph.14761
Franconi, F. & Campesi, I. (2014). Sex and gender influences on pharmacological response: an overview. Expert Review of Clinical Pharmacology, 7(4), 469-485. doi: 10.1586/17512433.2014.922866
Freire, A. C., Basit, A. W., Choudhary, R., Piong, C. W., Merchant, H. A. (2011). Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. International Journal of Pharmaceutics, 415(1-2), 15-28. https://doi.org/10.1016/j.ijpharm.2011.04.069.
Giefing-Kröll, C., Berger, P., Lepperdinger, G., Grubeck-Loebenstein, B. (2015). How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell, 14(3), 309-321. doi: 10.1111/acel.12326.
Greenwood, B. N., Carnahan, S., & Huang, L. (2018). Patient–physician gender concordance and increased mortality among female heart attack patients. Proceedings of the National Academy of Sciences, 115(34), 8569-8574.
Huded, C. P., Johnson, M., Kravitz, K., Menon V., Abdallah M., Gullett T. C...Khot U. N. (2018). Four-step protocol for disparities in stemi care and outcomes in women. Journal of the American College of Cardiology, 71(19), 2122-2132. doi: 10.1016/j.jacc.2018.02.039.
Juul, K.V., K. B. (2011). Gender difference in antidiuretic response to desmopressin. American Journal of Physiology-Renal Physiology, 300(5), F1116-22. doi: 10.1152/ajprenal.00741.2010.
Mastroianni, A. C., Faden, R., Federman, D. (Eds.). (1994). Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. (Vol. I). Washington, DC: National Academies Press.
Mauvais-Jarvis, F., Berthold, H. K., Campesi, I., Carrero, J. J., Dakal, S., Franconi, F., Gouni-Berthold, I., Heinman, M. L., Kautzky-Willer, A., Klein, S. L., Murphy, A., Regitz-Zagrosek, V., Reue, K., & Rubin, J. B. (2021). Sex-and Gender-Based Pharmacological Response to Drugs. Pharmacological Reviews, 73(2), 730-762.
Mazure, C. M., & Jones, D. P. (2015). Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Women's Health, 15(1). doi: 10.1186/s12905-015-0251-9.
Moga, D. C., Taipale, H., Tolppanen, A. M., Tanskanen, A., Tiihonen, J., Hartikainen, S…Gnjidic, D. (2017). A comparison of sex differences in psychotropic medication use in older people with alzheimer's disease in the US and Finland. Drugs Aging, 34(1), 55-65. doi: 10.1007/s40266-016-0419-5.
Morgan, S. G., Weymann, D., Pratt, B., Smolina, K., Gladstone, E. J., Raymond, C., Mintzes, B. (2016). Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age Ageing, 45(4), 535-42. doi: 10.1093/ageing/afw074.
Obias-Manno, D., Scott, P. E., Kaczmarczyk, J., Miller, M., Pinnow, E., Lee-Bishop, L… Uhl, K. (2007). The food and drug administration office of women's health: impact of science on regulatory policy. Journal of Women's Health, 16(6). https://doi.org/10.1089/jwh.2006.0135.
Rochon, P. A., Gruneir, A., Bell, C. M., Savage, R., Gill, S. S., Wu, W...Bronskill, S.E. Comparison of prescribing practices for older adults treated by female versus male physicians: a retrospective cohort study. (2018, Oct 22). Plos One, 13(10: e0205524). https://doi.org/10.1371/journal.pone.0205524.
Sheehan, C. M., & Tucker-Drob, E. M. (2017). Gendered expectations distort male–female differences in instrumental activities of daily living in later adulthood. The Journals of Gerontology: Series B, 74(4), 715-723.
Spoletini, I., Vitale, C., Malorni, W., Rosano, G. M. C. (2012). Sex differences in drug effects: interaction with sex hormones in adult life. In Regitz-Zagrosek, V. (Ed.), Sex and Gender Differences in Pharmacology. Handbook of Experimental Pharmacology (Vol. 14, pp. 91-105). Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-642-30726-3_5.
Sramek, J. J., Murphy, M. F., Cutler, N. R. (2016). Sex differences in the psychopharmacological treatment of depression. Dialogues in clinical neuroscience, 18(4), 447-457.
Tannenbaum C., Day D., Matera Alliance. (2017). Age and sex in drug development and testing for adults. Pharmacological Research, 121, 83-93. https://doi.org/10.1016/j.phrs.2017.04.027.
Tannenbaum, C., Greaves, L., Graham, I. D. (2016). Why sex and gender matter in implementation research. BMC Medical Research Methodology, 16(1), 145.
Tannenbaum, C., Lexchin, J., Tamblyn, R., Romans, S. (2009). Indicators for Measuring Mental Health: Towards Better Surveillance. Healthcare policy = Politiques de sante, 5(2), e177–e186.
US General Accounting Office. (2001). Drug Safety: Most Drugs withdrawn in Recent Years had Greater Health Risks for Women. Washington, DC: Government Publishing Office.
Verdam, M. G., Oort, F. J., & Sprangers, M. A. (2017). Item bias detection in the Hospital Anxiety and Depression Scale using structural equation modeling: comparison with other item bias detection methods. Quality of Life Research, 26(6), 1439-1450.
Welch, V. A., Norheim, O. F., Jull, J., Cookson, R., Sommerfelt, H., & Tugwell, P., & CONSORT-Equity and Boston Equity Symposium (2017). CONSORT-Equity 2017 extension and elaboration for better reporting of health equity in randomised trials. BMJ, 359 :j5085.
Welch, V., Petticrew, M., Tugwell, P., Moher, D., O'Neill, J., Waters, E…The PRISMA-Equity Bellagio group. (2012). PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLOS Medicine, 9(10: e1001333). https://doi.org/10.1371/journal.pmed.1001333.
WHO Gender Responsive Assessment Scale: criteria for assessing programmes and policies. In: WHO Gender Mainstreaming Manual for Health Managers: a practical approach. 2011. Available at: https://www.who.int/gender/mainstreaming/GMH_Participant_GenderAssessmentScale.pdf.
Historically, drug development has been conducted predominantly on males, from preclinical research in mice and rats to clinical trials. As a result, women report more unwanted, and sometime deadly, side effects than men.
Gendered Innovations:
1. Include Females and Males in all Stages of Drug Development. Females and males must be included in all drug trials—from early cell and animal testing to human clinical trials—and data must be disaggregated by sex and analyzed separately.
- • Age and genetic ancestry should also be considered.
- • Pregnancy also requires attention: pregnant women get sick, and sick women get pregnant.
- • Information about drug safety effects on the fetus is also a priority. Including females and males in drug development will help us understand sex differences in efficacy and allow for the development of sex-specific dosing or treatments, where necessary.
- • Gender and age bias can influence diagnostic questions.
- • Gender relations between the healthcare provider and the patient can affect prescribing patterns and treatment outcomes.
- • Age and race can also play a role. Promoting gender-sensitive treatments requires eliminating gender bias and stereotypes in diagnostic questions, allowing for better detection of symptoms in women, men, and gender-diverse people.