環境化学物質:保健・生物医学分野の研究デザイン
要旨
ジェンダード・イノベーション・プロジェクトは、欧州委員会から、第7次欧州研究開発フレームワーク計画(FP7)のいくつかのプロジェクトを分析するよう依頼された。このケーススタディでは、「女性における環境化学物質の生殖への影響」(REEF)プロジェクトについて取り上げる。性差分析によるイノベーション、セックス/ジェンダー分析の手法、そしてジェンダード・イノベーション手法の活用によって「付加価値」が生まれる可能性がある点について明らかにする。
課題:
環境化学物質がヒトの生殖の健康に及ぼす潜在的影響については、これまで主に男性を対象として研究が行われてきた。世界保健機関(WHO)に対して、専門家グループは「女性の生殖機能の発達も内分泌の干渉を受けやすい」という事実があるにもかかわらず、「女性の生殖機能の健康の変化傾向については、男性と比較してはるかに研究が進んでいない」と報告している(Damstra et al., 2002,)。
方法:保健・生物医学研究のデザイン
研究者たちは、環境化学物質が女性の生殖機能に及ぼす影響に関する科学的知見の空白を埋めることを目的として、実験を計画した。対照実験で動物のメスをサンプリングすることにより、環境化学物質への曝露の生理的および遺伝的影響に関する新しい知見が得られた。また、研究者は妊娠中のメスもサンプリングしており、妊娠結果を評価項目に用いることが可能になっている。さらに、妊娠中のメスを研究することで、環境化学物質の胎内曝露が女性および男性の胎児に及ぼす影響を調査し、感受性における性差を解明するという貴重な研究成果が得られている。
ジェンダード・イノベーション:
- 1. REEFプロジェクトは、環境中の化学物質への曝露が妊娠中の女性およびその男性・女性の子孫に与える影響に焦点を当てたプロジェクトである。
- 2. REEFプロジェクトでは、性差の分析を行うことで、ヒトを含め、雌雄それぞれに対する胎内での環境化学物質の影響を比較した。さらに、出生後の動物を研究することは、ヒトにおける環境化学物質の影響のモニタリングにも役立つ可能性がある。
ジェンダード・イノベーション手法による研究への付加価値の可能性
- 1. 妊娠と環境化学物質の影響の潜在的相互作用の調査
- 2. 環境化学物質のヒトへの影響における、性差、年齢、職業、地理的位置、社会
Background: The European Union Framework Programme 7 (FP7) Reproductive Effects of Environmental Chemicals in Females (REEF) Project
Gendered Innovation 1: Studying the Effects of Environmental Chemicals (ECs) in Pregnant Females and Their Offspring
Method: Designing Health and Biomedical Research
Gendered Innovation 2: Comparing EC Effects in Females and Males
Method: Analyzing Sex
Potential Value Added to Future Research through the Future Application of Gendered Innovations
Methods
Value Added 1: Investigating Possible Interactions between Pregnancy and EC Effects
Value Added 2: Understanding the Influence of Sex, Age, Occupation, Geographic Location, Socioeconomic Status, and Body Composition in Potential EC Effects on Humans
Method: Intersectional Approaches
Conclusions
Works Cited
Background
The REEF project is a study of the effects of environmental chemicals (ECs, including endocrine disruptors) on mammals, with the potential to improve human health by identifying possible risks posed by ECs. REEF researchers' "ultimate concern is to determine how the developing human female fetus might be affected by exposure to ECs" (REEF, 2009b). This project also studies male fetuses (Fowler et al., 2008). The diagram below shows relationships between a subset of REEF experiments:
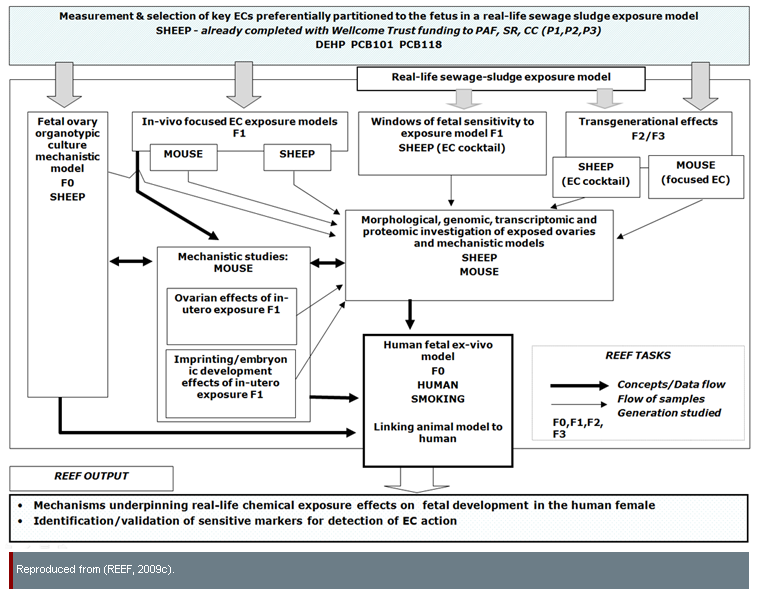
In "EC cocktail" experiments, environmentally-relevant EC doses were obtained using a cohort of sheep grazing on sewage-sludge-treated pastures; these animals were exposed to a complex mixture of compounds which may mirror human exposure patterns better than laboratory experiments involving a single test chemical (NECTAR, 2009). The effects of ECs may be additive—that is, exposure to two or more chemicals at given concentrations may produce adverse effects, even if exposure to any single chemical at the same concentration would not be harmful. Further, effects may be synergistic—that is, the effects of exposure to two or more chemicals may be greater or different than the simple sum of their effects in isolation (Kortenkamp et al., 2011; Crews et al., 2003).
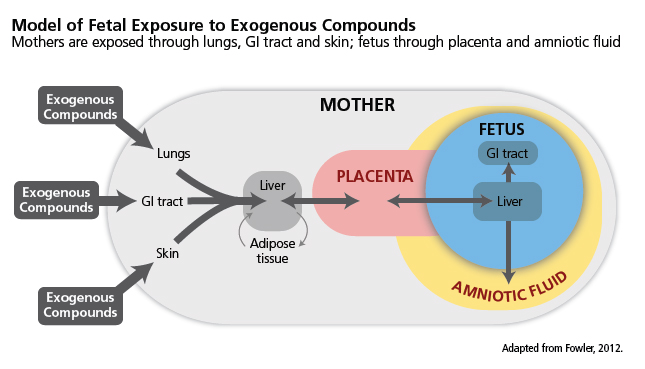
In the context of this case study, EC exposure includes intentional exposure (for example, though tobacco smoking)—see diagram.
Gendered Innovation 1: Studying the Effects of ECs in Pregnant Females and Their Offspring
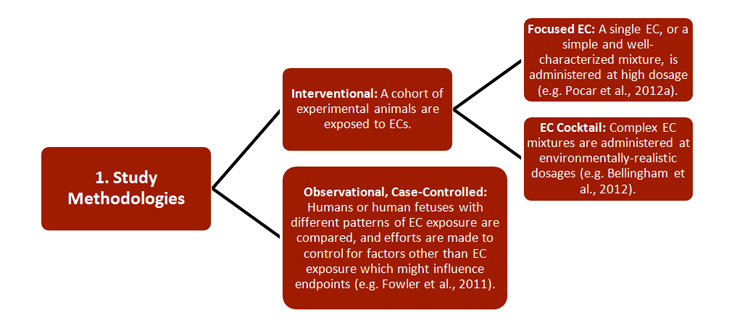
REEF has developed new strategies for studying the effects of ECs on both pregnant females and their offspring, with relevance to environmental monitoring and legislation. Researchers combined a wide range of established approaches, including 1) methodologies, 2) research subjects, 3) generational cohorts, 4) exposure routes, and 5) endpoints—see chart.
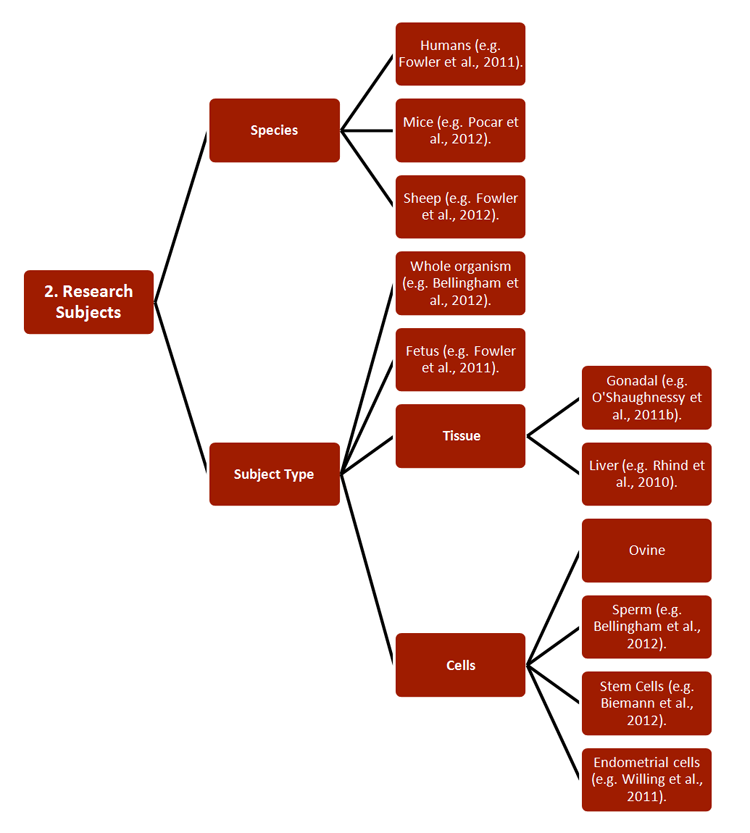
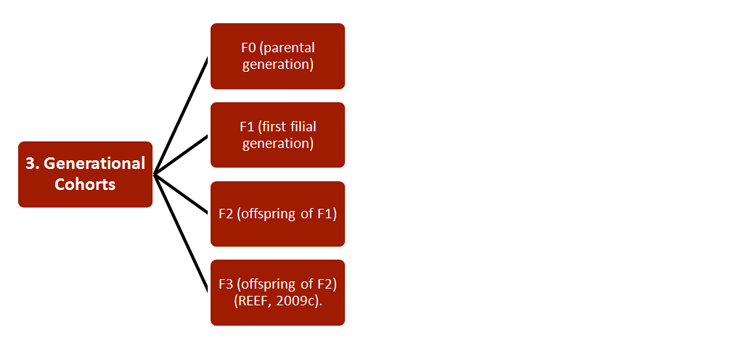
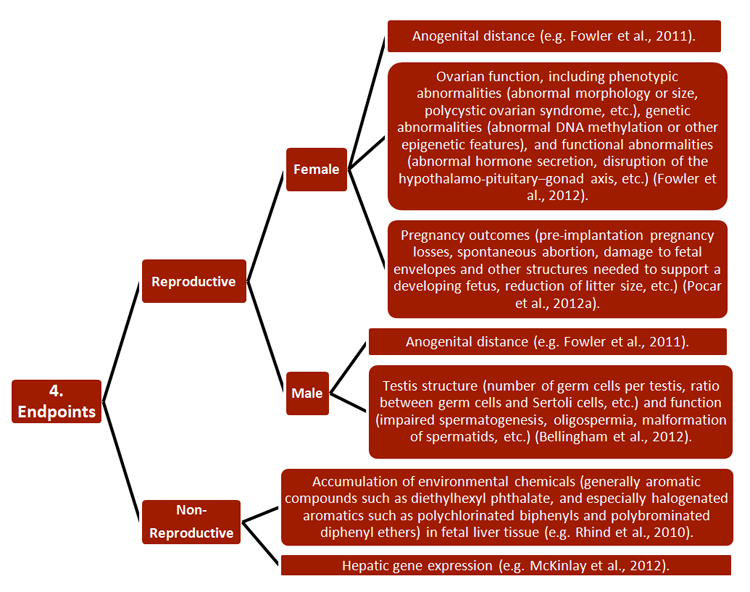

Studies have also increased basic understanding of pregnancy, particularly of the functions and epigenetics of endometrial tissue (Sandra et al., 2010). Certain of these findings are described in Gendered Innovation 2.
Method: Designing Health and Biomedical Research
REEF researchers designing experiments using sheep, mice, and humans have sampled both females and males, as well as pregnant and non-pregnant females (Pocar et al., 2012a; Pocar et al., 2012b). This design allows detection of sex-specific effects of chemical exposure (for example, ovarian atresia in females and poor sperm quality in males). Further, it allows sex differences in EC effects to be identified or ruled out.
Gendered Innovations 2: Comparing EC Effects in Females and Males
REEF researchers have identified similarities and differences in the effects of ECs on female and male animals. These include:
-
1. Sex similarities and differences in EC effects on bone homeostasis: Ewes and rams grazing on EC-contaminated pastures fertilized with sewage sludge similarly display defects in bone homeostasis, but "the bone tissue is more sensitive in rams than in ewes to pollutants present in sewage sludge." In particular, rams show increases in bone mineral density, decreases in cross-sectional area, and shrinking of the marrow space to a greater degree than ewes (Lind et al., 2009).
- 2. Sex differences in the inter-generational transmission of EC effects: When pregnant mice are exposed to a given concentration of polychlorinated biphenyls (PCBs), their offspring will show sex-specific developmental defects-for example, follicular atresia in females and reduced sperm viability in males. However, the heritability of these effects differs: only first-generation females are affected, but males are affected up to the third generation (Pocar et al., 2012b). Pregnant females in this experiment were fed up to 100 μg/kg/day of a mixture of PCB congeners 101 and 118. Both congeners have been assigned toxicity equivalence factors (TEFs) of 0.00003 (U.S. EPA, 2009; Van den Berg et al., 2005). Therefore, this exposure corresponds to 0.03 μg toxic equivalent (TEQ) /kg/day, or 30,000 pg TEQ/kg/day, which is far higher than typical human intakes, estimated at 1 pg TEQ/kg/day (Judd et al., 2004).
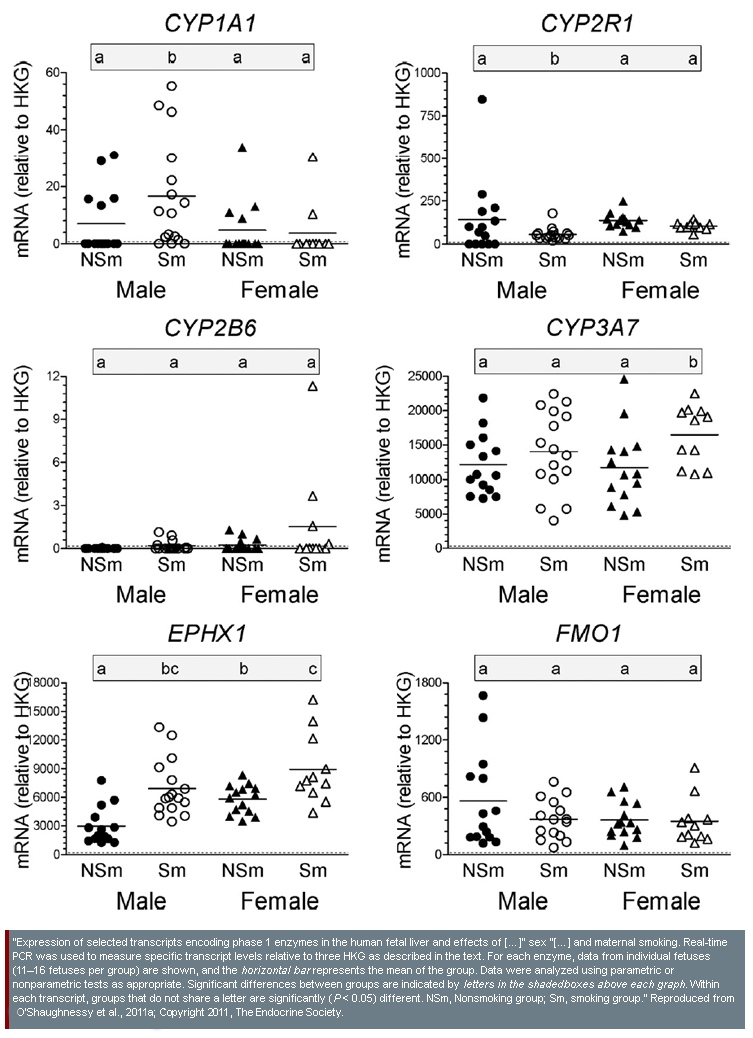
- 3. Sex similarities and differences in possible EC effects on human fetal liver development: Researchers examining mRNA transcription and protein expression in the fetal liver found differences between fetuses from mothers who smoked tobacco and fetuses from non-smoking mothers. In some cases, the observed effect was the same in smoke-exposed fetuses of both sexes; both expressed higher levels of EPHX1 than non-exposed fetuses. In other cases, observed effects were different by sex; smoke-exposed male fetuses showed depressed expression of GGT1, CYP2R1, and CAR, whereas no genes were observed to be depressed in smoke-exposed female fetuses (O'Shaughnessy et al., 2011a)—see diagram.
Method: Analyzing Sex
By studying animals of both sexes, researchers identified both similarities and differences in EC effects (Pocar et al., 2012a; Lind et al., 2009). Many REEF studies have utilized both females and males in a given experiment, avoiding the difficulties in evaluating sex differences through meta-analysis of single-sex studies (Fowler et al., 2011; O'Shaughnessy et al., 2011a).
Potential Value Added to Future Research through the Future Application of Gendered Innovations Methods
Value Added 1: Investigating Possible Interactions between Pregnancy and EC Effects
Researchers may gain additional knowledge by analyzing factors intersecting with sex. For females, pregnancy may be an important determinant of EC exposure and its consequences. Many REEF studies have compared male animals to non-pregnant females, and other studies have examined pregnant females in detail, but no studies specifically compare pregnant and non-pregnant females. Such comparisons could provide information about pregnancy's effects on absorption, mobilization, and metabolism of ECs.
Value Added 2: Understanding the Influence of Sex, Age, Occupation, Geographic Location, Socioeconomic Status, and Body Composition in Potential EC Effects on Humans
If the REEF project is expanded to include epidemiological studies of human populations, many variables apart from sex may be important to understanding EC exposures and effects.
Method: Intersectional Approaches
Variables to consider include:
- 1. Age at Exposure: While adults are mainly exposed to ECs through foods, children may consume such substances when they put their fingers in their mouths (Sexton et al., 2004). Airborne ECs (gases, aerosols, and particulates) tend to be most concentrated near the ground, and so children’s shorter height may increase their risk of EC inhalation (Moya et al., 2004). Age influences the consequences of exposure as well as patterns of exposure. ECs may produce different effects on developing fetuses, infants, pre-pubescent children, and adults (Barker, 2004).
Age may interact with sex. For example, among people exposed to inorganic arsenic through drinking water, women metabolize highly-toxic inorganic arsenic into less-toxic dimethylarsenic compounds significantly more efficiently than men. This observed sex difference interacts with age; women's metabolic advantage disappears after menopause (Lindberg et al., 2008).
- 2. Occupation: Gendered divisions in labor influence exposure to ECs in agriculture. In areas where farming is mechanized, such as in the U.S. and Europe, the majority of agricultural workers are men (U.S. Department of Labor Employment and Training Association, 2010; NationMaster Labor Statistics, 2010). In these countries, most occupational exposure to pesticides occurs in men (Rusiecki et al., 2004). In Africa, by contrast, 75% of agricultural workers are women (London et al., 2002; NationMaster Labor Statistics, 2010). Gender inequalities contribute to other problems: women in developing countries are often less educated than men and hence less able to read pesticide label warnings. In many countries, women were also less likely than men to have access to protective equipment such as gloves and face masks (London et al., 2002).
Gender roles influence patterns of both paid and unpaid labor, and therefore may influence the risk of EC exposures through domestic work (Snijder et al., 2012). Recent studies suggest that women perform between 65% and 75% of all domestic housecleaning in the United States (Coltrane, 2000). Housecleaning is a potential source of exposure to Nonylphenol, as “nonylphenol […] and its ethoxylates are used widely as surfactants in industrial and household cleaners” (Wilson et al., 2007). However, existing studies of nonylphenol levels in urine show no significant sex differences (Calafat et al., 2005).
- 3. Socio-Economic Status: Measures of socio-economic status, such as household income, correlate in some cases with measures of EC exposure. For example, among U.S. residents, urinary concentrations of triclosan, "a synthetic chemical with broad antimicrobial activity that has been used extensively in consumer products, including personal care products, textiles, and plastic kitchenware," are highest in people with the highest household incomes (Calafat et al., 2008a). Another class of environmental chemicals—the plasticizers Bisphenol A and 4-tertiary-Octylphenol—show the opposite pattern, with urinary concentrations highest in low-income groups (Calafat et al., 2008b). Low socio-economic status may increase exposure to pollutants—for example, in the U.S., people with low incomes are disproportionately likely to live in undesirable areas with high levels of pollution—for example, areas surrounding trash incinerators (Mohai et al., 2009). Researchers in England investigated the relationship between environmental pollution in a given area and the socio-economic status of residents. The relationships involved are complex, and trends observed are sensitive to ways of measuring both pollution and wealth (Briggs et al., 2008).
- 4. Geographic Location: Location can determine exposure to environmental chemicals—for example, people living near agricultural areas may be exposed to pesticides through aerial spraying, even if they are not agricultural workers (Ward et al., 2000).
- 5. Body Composition: Adults with higher BMIs in the United States have significantly higher concentrations of BPA in their urine than people with lower BMIs, and BPA levels are correlated with BMI over a wide range (Lang et al., 2008). Though it is well established that BPA exposure can cause obesity in animal models, people who are obese may also accumulate higher BPA levels because the compound—like many ECs—is lipophilic (Vandenberg et al., 2007).
Conclusions:
EC effects cannot be fully understood in females or males without studying pregnancy and fetal development, due to the unique characteristics of the in utero environment and the susceptibility of fetuses to certain forms of EC exposure. A key objective of REEF researchers is "to integrate the mouse and sheep exposure studies with […] human" studies (REEF, 2009b). Such integration requires consideration of social factors—such as gender roles and socioeconomic status—in addition to biological factors such as age, sex, and body composition.
Works Cited:
Bakker, R. (2011). Maternal Lifestyle and Pregnancy Complications: The Generation R Study. Rotterdam: Optima Grafische Communicatie.
Barker, D. (2004). The Developmental Origins of Adult Disease. Journal of the American College of Nutrition, 23 (S6), 588S-595S.
Barbeau, E., Krieger, N., & Soobader, M. (2004). Working Class Matters: Socioeconomic Disadvantage, Race/Ethnicity, Gender, and Smoking in the National Health Information Survey (NHIS) 2000. American Journal of Public Health, 94 (2), 269-278.
Bautier, P. (2006). International Women's Day: A Statistical View of the Life of Women and Men in the EU25. Luxembourg: Eurostat Press Office
Bellingham, M., McKinnell, C., Fowler, P., Amezaga, M., Zhang, Z., Rhind, M., Cotinot, C., Mandon-Pepin, B., Evans, N., & Sharpe, R. (2012). Foetal and Post-Natal Exposure of Sheep to Sewage Sludge Chemicals Disrupts Sperm Production in Adulthood in a Subset of Animals. International Journal of Andrology, (epub ahead of print).
Biemann, R., Santos, A., Santos, A., Riemann, D., Knelangen, J., Blüher, M., Koch, H., & Fischer, B. (2012). Endocrine Disrupting Chemicals Affect the Adipogenic Differentiation of Mesenchymal Stem Cells in Distinct Ontogenetic Windows. Biochemical and Biophysical Research Communications, 417 (2), 747-752.
Boyle, P., & Ferlay, J. (2004). Cancer Incidence and Mortality in Europe, 2004. Annals of Oncology, 16 (3), 481-488.
Briggs, D., Abellan, J., & Fecht, D. (2008). Environmental Inequality in England: Small Area Associations between Socio-Economic Status and Environmental Pollution. Social Science in Medicine, 67 (10), 1612-1629.
Calafat, A., Ye, X., Wong, L., Reidy, J., & Needham, L. (2008a). Urinary Concentrations of Triclosan in the U.S. Population: 2003-2004. Environmental Health Perspectives, 116 (3), 303-307.
Calafat, A., Ye, X., Wong, L., Reidy, J., & Needham, L. (2008b). Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environmental Health Perspectives, 116 (1), 39-44.
Calafat, A., Wong, L., Kuklenyik, Z., Reidy, J., & Needham, L. (2007). Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environmental Health Perspectives, 115 (11), 1596-1602.
Calafat, A., Kuklenyik, Z., Reidy, J., Caudil, S., Ekong, J., & Needham, L. (2005). Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environmental Health Perspectives, 113 (4), 391-395.
Coltrane, S. (2000). Research on Household Labor: Modeling and Measuring the Social Embeddedness of Routine Family Work. Journal of Marriage and Family, 62 (4), 1208-1233.
Crews, D., Putz, O., Thomas, P., Hayes, T., & Howdeshell, K. (2003). Wildlife as Models for the Study of how Mixtures, Low Doses, and the Embryonic Environment Modulate the Action of Endocrine-Disrupting Chemicals. Pure and Applied Chemistry, 75 (11-12), 2305-2320.
Damstra, T., Barlow, S., Bergman, A., Kavlock, R., & Van der Kraak, G. (2002). Global Assessment of the State-of-the-Science of Endocrine Disruptors. Geneva: World Health Organization (WHO).
Ferlay, J., Autier, P., Boniol, M., Heanue, M., Colombet, M., & Boyle, P. (2006). Estimates of the Cancer Incidence and Mortality in Europe in 2006. Annals of Oncology, 18 (3), 581-592.
Fowler, P., Bellingham, M., Sinclair, K., Evans, N., Pocar, P., Fischer, B., Schaedlich, K., Schmidt, J., Amezaga, M., Bhattacharya, S., Rhind, S., & O’Shaughnessy, P. (2012). Impact of Endocrine-Disrupting Compounds (EDCs) on Female Reproductive Health. Molecular and Cellular Endocrinology, 355 (2), 231-239.
Fowler, P. (2012). REEF (Reproductive Effects of Environmental Chemicals in Females): Presentation at the Gendered Innovations Expert's Meeting, May 18th-19th, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid.
Fowler, P., Bhattacharya, S., Flannigan, S., Drake, A., & O'Shaughnessy, P. (2011). Maternal Cigarette Smoking and Effects on Androgen Action in Male Offspring: Unexpected Effects on Second-Trimester Anogenital Distance. Journal of Clinical Endocrinology and Metabolism, 96 (9), E1502-E1506.
Fowler, P., Cassie, S., Rhind, S., Brewer, M., Collinson, J., Lea, R., Baker, P., Bhattacharya, S., & O'Shaughnessy, P. (2008). Maternal Smoking during Pregnancy Specifically Reduces Human Fetal Desert Hedgehog Gene Expression during Testis Development. Journal of Clinical Endocrinology and Metabolism, 93 (2), 619-626.
Judd, N., Griffith, W., & Faustman, E. (2004). Contribution of PCB Exposure from Fish Consumption to Total Dioxin-Like Dietary Exposure. Regulatory Toxicology and Pharmacology, 40 (2), 125-135.
Karim-Kos, H., de Vries, E., Soerjomataram, I., Lemmens, V., Siesling, S., Willem, J., & Coebergh, W. (2008). Recent Trends of Cancer in Europe: A Combined Approach of Incidence, Survival, and Mortality for 17 Cancer Sites since the 1990s. European Journal of Cancer, 44 (10), 1345-1389.
Katalinic, A., & Rawal, R. (2007). Decline in Brease Cancer Incidence after Decrease in Utilization of Hormone Replacement Therapy. Breast Cancer Research and Treatment, 107 (3), 427-430.
Kortenkamp, A., Martin, O., Faust, M., Evans, R., McKinlay, R., Orton, F., & Rosivatz, E. (2011). State of the Art Assessment of Endocrine Disrupters: Final Report. Brussels: European Commission.
Lang, I., Galloway, T., Scarlett, A., Henley, W., Depledge, M., Wallace, R., Mezler, D. (2008). Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. Journal of the American Medical Association, 300 (11), 1303-1310.
Lindberg, A., Ekström, E., Nermell, B., Rahman, M., Lönnerdal, B., Persson, L., & Vahter, M. (2008). Gender and Age Differences in the Metabolism of Inorganic Arsenic in a Highly Exposed Population in Bangladesh. Environmental Research, 106 (1), 110-120.
Lind, P., Gustafsson, M., Hermsen, S., Larsson, S., Kyle, C., Orberg, J., & Rhind, S. (2009). Exposure to Pastures Fertilized with Sewage Sludge Disrupts Bone Tissue Homeostasis in Sheep. Science of the Total Environment, 407 (7), 2200-2208.
London, L., de Grosbois, S., Wesseling, C., Kisting, S., Rother, H., & Mergler, D. (2002). Pesticide Usage and Health Consequences for Women in Developing Countries: Out Of Sight, Out Of Mind? International Journal of Occupational Health, 8 (1), 46-59.
Luebker, D., York, R., Hansen, K., Moore, J., & Butenhoff, J. (2005). Neonatal Mortality from in utero Exposure to Perfluorooctanesulfonate (PFOS) in Sprague–Dawley rats: Dose–Response, and Biochemical and Pharamacokinetic Parameters. Toxicology, 215 (1-2), 149-169.
McKinlay, R., O'Shaughnessy, P., Sharpe, R., & Fowler, P. (2012). In-Utero Exposure to Environmental Chemicals: Lessons from Maternal Cigarette Smoking and its Effects on Gonad Development and Puberty. In Diamantia-Kandarakis, E., & Gore, A. (Eds.), Endocrine Disruptors and Puberty, pp. 11-48. London: Humana Press.
Mohai, P., Pellow, D., & Roberts, J. (2009). Environmental Justice. Environment and Resources, 34, 405-130.
Moya, J., Bearer, C., & Etzel, R. (2004). Children’s Behavior and Physiology and How it Affects Exposure to Environmental Contaminants. Pediatrics, 113 (S3), 996-1006.
NationMaster Labor Statistics. (2010). Labor Statistics By Country, Agricultural Workers, Percent Female, Most Recent. Rapid Intelligence Online.
Network for Environmental Chemical Toxicants Affecting Reproduction (NECTAR). (2010). About.
Network for Environmental Chemical Toxicants Affecting Reproduction (NECTAR). (2009). NECTAR Cluster.
O'Shaughnessy, P., Monteiro, A., Bhattacharya, S., & Fowler, P. (2011a). Maternal Smoking and Fetal Sex Significantly Affect Metabolic Enzyme Expression in the Human Fetal Liver. Journal of Clinical Endocrinology and Metabolism, 96 (9), 2851-2860.
O'Shaughnessy, P., Monteiro, A., & Fowler, P. (2011b). Identification of Stable Endogenous Reference Genes for Real-Time PCR in the Human Fetal Gonad using an External Standard Technique. Molecular Human Reproduction, 17 (10), 620-625.
O'Shaughnessy, P., Monteiro, A., Bhattacharya, S., & Fowler, P. (2011a). Supplemental Data (Supplemental Table 1 and Supplemental Figures 1-2) — Maternal Smoking and Fetal Sex Significantly Affect Metabolic Enzyme Expression in the Human Fetal Liver. Journal of Clinical Endocrinology and Metabolism, 96 (9), 2851-2860.
Pocar, P., Fiandanese, N., Secchi, C., Berrini, A., Fischer, B., Schmidt, J., Schaedlich, K., & Borromeo, V. (2012a). Exposure to Di (2-Ethyl-Hexyl) Phthalate (DEHP) in Utero and During Lactation Causes Long-Term Pituitary-Gonadal Axis Disruption in Male and Female Mouse Offspring. Endocrinology, 153 (2), 937-948.
Pocar, P., Fiandanese, N., Secchi, C., Berrini, A., Fischer, B., Schmidt, J., Schaedlich, K., Rhind, S., Zhang, Z., & Borromeo, V. (2012b). Effects of Polychlorinated Biphenyls in Cd-1 Mice: Reproductive Toxicity and Intergenerational Transmission. Toxicological Sciences, 126 (1), 213-226.
Reproductive Effects of Environmental Chemicals in Females (REEF) Project. (2009a). Summary.
Reproductive Effects of Environmental Chemicals in Females (REEF) Project. (2009b). Objectives.
Reproductive Effects of Environmental Chemicals in Females (REEF) Project. (2009c). Program Evaluation Review Technique (PERT) Diagram.
Rhind, S., Kyle, C., Mackie, C., McDonald, L., Zhang, Z., Duff, E., Bellingham, M., Amezaga, M., Mandon-Pepin, B., Loup, B., Cotinot, C., Evans, N., Sharpe, R., & Fowler, P. (2010). Maternal and Fetal Tissue Accumulation of Selected Endocrine-Disrupting Compounds (EDCs) Following Exposure to Sewage Sludge-Treated Pastures Before or After Conception. Journal of Environmental Monitoring, 12 (8), 1582-1593.
Rhind, S., Kyle, C., Mackie, C., & McDonald, L. (2009). Accumulation of Endocrine Disrupting Compounds in Sheep Fetal and Maternal Liver Tissue following Exposure to Pastures Treated with Sewage Sludge. Journal of Environmental Monitoring, 11 (8), 1469-1476.
Rusiecki, J., De Roos, A., Lee, W., Dosemeci, M., Lubin, J., Hoppin, J., Blair, A., & Alavanja, M. (2004). Cancer Incidence among Pesticide Applicators Exposed to Atrazine in the Agricultural Health Study. Journal of the National Cancer Institute, 96 (18), 1375-1382.
Sandra, O., Mansouri-Attia, N., & Lea, R. (2010). Novel Aspects of Endometrial Function: A Biological Sensor of Embryo Quality and Driver of Pregnancy Success. Reproduction, Fertility, and Development, 24 (1), 68-79.
Schadt, E., Molony, C., Chudin, E., Hao, K., Yang, X., Lum, P., Kasarskis, A., Zhang, B., Wang, S., Suver, C., Zhu, J., Millstein, J., Sieberts, S., Lamb, J., Guha-Thakurta, D., Derry, J., Storey, J., Avila-Campillo, I., Kruger, M., Johnson, J., Rohl, C., van Nas, A., Mehrabian, M., Drake, T., Lusis, A., Smith, R., Guengerich, F., Strom, S., Schuetz, E., Rushmore, T., & Ulrich, U. (2008). Mapping the Genetic Architecture of Gene Expression in Human Liver. Public Library of Science (PLoS) Biology, 6 (5), e107.
Sexton, K., Needham, L., & Pirkle, J. (2004). Human Biomonitoring of Environmental Chemicals. American Scientist, 92 (1), 38-45.
Snijder, C., Roeleveld, N., Te Velde, E., Steegers, E., Raat, H., Hofman, A., Jaddoe, V., & Burdorf, A. (2012). Occupational Exposure to Chemicals and Fetal Growth: The Generation R Study. Human Reproduction, 27 (3), 910-920.
Soerjomataram, I., Pukkala, E., Brenner, H., Willem, J., & Coebergh, W. (2008). On the Avoidability of Breast Cancer in Industrialized Societies: Older Mean Age at First Birth as an Indicator of Excess Breast Cancer Risk. Breast Cancer Research and Treatment, 111 (2), 297-302.
United States Department of Labor Employment and Training Administration (ETA). (2010). The National Agricultural Worker's Survey. Washington, D.C.: Government Publishing Office (GPO).
United States Environmental Protection Agency (U.S. EPA). (2009). Recommended Toxicity Equivalency Factors (TEFs) for Human Health Risk Assessments of Dioxin and Dioxin-Like Compounds: External Review Draft. Washington, D.C.: Government Publishing Office (GPO).
Vandenberg, L., Hauser, R., Marcus, M., Olea, N., & Welshons, W. (2007). Human Exposure to Bisphenol A (BPA). Reproductive Toxicology, 24 (2), 139-177.
Van den Berg, M., Birnbaum, L., Denison, M., De Vito, M., Farland, W., Feeley, M., Fiedler, H., Hakansson, H., Hanberg, A., Haws, L., Rose, M., Safe, S., Schrenk, D., Tohyama, C., Tritscher, A., Tuomisto, J., Tysklind, M., Walker, N., & Peterson, E. (2006). The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicological Sciences, 93 (2), 223-241.
Ward, M., Nuckols, J., Weigel, S., Maxwell, S., Cantor, K., & Miller, R. (2000). Identifying Populations Potentially Exposed to Agricultural Pesticides using Remote Sensing and a Geographic Information System. Environmental Health Perspectives, 108 (1), 5-12.
Willing, C., Peich, M., Danescu, A., Kehelen, A., Fowler, P., & Hombach-Klonisch, S. (2011). Estrogen-Independent Actions of Environmentally Relevant AhR-Agonists in Human Endometrial Epithelial Cells. Molecular Human Reproduction, 17 (2), 115-126.
Wilson, N., Chuang, J., Morgan, M., Lordo, R., & Sheldon, L. (2007). An Observational Study of the Potential Exposures of Preschool Children to Pentachlorophenol, Bisphenol-A, and Nonylphenol at Home and Daycare. Environmental Research, 103 (1), 9-20.
The Gendered Innovations project was asked by the European Commission to analyze several of its Framework Programme 7 (FP7) projects. The Reproductive Effects of Environmental Chemicals in Females (REEF) project studies the effects of environmental chemicals (including endocrine disruptors) on the developing human fetus. We identify gendered innovations, methods of sex and gender analysis, and points of potential "value added" through the future application of gendered innovations methods.
Gendered Innovation:
REEF samples both females and males, as well as pregnant and non-pregnant females to detect sex-specific effects of chemical exposure (for example, ovarian atresia in females and poor sperm quality in males) and to identify or rule out sex differences. This research design has allowed REEF researchers to identify, for example, a greater negative impact of environmental chemicals on male bone health in sheep (compared to females).
Potential Value Added to Future Research through the Application of Gendered Innovations Methods:
Researchers may gain additional knowledge by Analyzing Factors Intersecting with Sex. For females, pregnancy may be an important determinant of environmental chemical exposure and its consequences. Many REEF studies have compared male animals to non-pregnant females, and other studies have examined pregnant females in detail, but no studies specifically compare pregnant and non-pregnant females. Such comparisons may provide information about pregnancy's effects on absorption, mobilization, and metabolism of environmental chemicals.